Stanford researchers map electric fields to help unravel how enzymes work
Probe developed by Stanford researchers reveals previously unknown structure of electric fields inside an enzyme’s active site, yielding clues to source of enzymes’ power.
Every moment in our bodies' cells, countless activities vital to life occur thanks to enzymes. These special proteins act as catalysts by accelerating the pace and improving the selectivity of chemical reactions without undergoing permanent changes themselves. Beyond their indispensable role in biology, enzymes are also critical for myriad processes in the food, pharmaceutical, agriculture, and cosmetics industries.
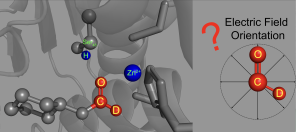
Belying their ubiquity and importance, enzymes are poorly understood. In particular, scientists want to know what makes enzymes’ active sites—the pocket-like region where the sped-up chemical reactions take place—so powerful. While the three-dimensional atomic structures of many enzymes' active sites have been visualized and mapped, the "invisible" structure of the electric fields inside an active site is mostly unknown. Those electric fields are theorized to play an important role in forming a precise environment in active sites where molecules react and rapidly transition to new molecules.
Now a study co-led by Stanford researchers Chu Zheng and Yuezhi Mao has debuted a new probe for measuring and visualizing the electric fields inside an enzyme’s active site. The paper, recently published in the journal Nature Chemistry, reports on the orientation of electric fields at the site of the reaction and could help researchers calculate the key chemical interactions in active sites. These insights, in turn, could lead to building custom-tailored synthetic enzymes for industry, as well as greatly advancing the discovery and design of new drugs that interfere with or modulate the function of enzyme targets.
"We have developed a novel probe that can give us important information about how electric fields are uniquely oriented in enzymes, which we think is fundamental to the amazing catalytic power of enzymes," said Zheng, a graduate student in the lab of Steven G. Boxer, the Camille Dreyfus Professor of Chemistry.
"On a basic level, we are trying to better understand how enzymes work, and in this study, we are adding a new dimension by bringing in electric field orientations which is believed to have a critical impact on enzyme's catalytic functions,” said Mao, a postdoctoral scholar in chemistry who works in the lab of Thomas Markland, an associate professor of chemistry at Stanford and also a senior co-author.
A potent new tool
The Boxer lab at Stanford has pioneered the concept of interpreting the functionality of enzymes by measuring electrostatic interactions, which are present in all forms of matter and are specifically organized in three dimensions in large biological molecules.
“The origin of the amazing functionality of enzymes is a general question, and it applies not just to biological catalysis but chemical catalysis as well—which is a huge business,” Boxer said. “Roughly 80 percent of all chemicals are made using catalysts, but what is actually responsible for lowering the activation free energy [to make the reaction occur faster] is not well understood for most reactions. Investigating the role of electric fields in enzyme function is very much at the heart of our work,” said Boxer, the chair of the Department of Chemistry at Stanford’s School of Humanities and Sciences and a senior co-author of the study.
The probe developed by the Stanford team relies on a technique—also developed in the Boxer lab—called vibrational Stark effect spectroscopy. This technique measures the vibrational frequencies in probe molecules based on the wavelength of infrared light absorbed by its chemical bonds. Shifts in these vibrational frequencies reveal information about the electric fields present. In this study, the researchers investigated shifts in the vibrational frequencies of chemical bonds in a probe made from a molecule called N-cyclohexylformamide. This molecule acts as an inhibitor, binding to the active site of an enzyme called liver alcohol dehydrogenase.
To visualize the electric field in the active site of liver alcohol dehydrogenase, the researchers targeted two bonds in the N-cyclohexylformamide probe about 120 degrees away from each other. That specific angle between the two bonds allowed the researchers to gauge not only the strength, or magnitude, of the electric field, but also the field's orientation. Previous studies from the Boxer lab on other enzyme active sites had reported on the magnitude of electric fields but not on their directions.
"We call this tool a two-directional probe because with this probe we can measure the electric field in an active site in two different directions," Zheng said. "Using the probe this way, we can reconstruct and extract the orientation information about the electric field. That hasn't been done in the past."
Gathering this key measurement first required some chemical sleight of hand. One of the N-cyclohexylformamide probe’s chemical bonds—between a carbon atom and a hydrogen atom—is notoriously difficult to observe in protein environments. So, the researchers swapped the hydrogen atom for the element's heavier cousin, called deuterium. The new carbon–deuterium bond proved amenable to measurement, and helped the researchers reveal the orientation of the electric field.
A precise enzymatic environment
The Stanford researchers combined their experimental data with computer simulations and quantum mechanical calculations to describe the electric field's interactions with N-cyclohexylformamide, modified with deuterium, at the active site of liver alcohol dehydrogenase. Those properties were then compared to the electric fields found in water, acetone, and other common solvents.
Notably, the researchers found the orientation of the electric field in the active site of liver alcohol dehydrogenase differs considerably from the electric field orientation in the solvents they studied. That result supports the idea that enzyme active sites feature what scientists call a preorganized electrostatic environment, or one in which the precise positioning of amino acids and the electrostatic environment they create help reduce the energy required for a chemical reaction to take place. This could be a key to enzymes' remarkable ability to catalyze reactions.
"With this study, we are helping to advance the concept of correlating the performance of enzymes with both the magnitude and orientation of the electric fields in active sites," Mao said. "What we have found is evidence that electric fields in the enzyme active sites are preorganized, and that is an important clue in solving the mystery of why enzymes have their amazing abilities."
The probe developed by the Stanford researchers could be used to investigate many other enzymes' active sites. Broadening knowledge in this way will bring scientists and engineers closer to being able to design bespoke enzymes with spectacular new characteristics.
"The ultimate goal of this research is to enable us to design enzymes that have superb catalytic performance for biomedical and industrial application," Zheng said. "We are still far from that, but we are making progress and have a better understanding now than before regarding how enzymes work."
This research was supported by the Stanford Center for Molecular Analysis and Design Fellowship; the German Research Foundation; the National Institutes of Health; the National Science Foundation; and the Camille Dreyfus Teacher-Scholar Awards Program. Use of the Stanford Synchrotron Radiation Lightsource (SSRL) at the Stanford Linear Accelerator Center National Accelerator Laboratory is supported by the U.S. Department of Energy. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research. This research also used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility operated by the Stanford Research Computing Center.