Striking rare gold: Stanford researchers unveil new material infused with gold in an exotic chemical state
A form of gold that does not occur stably in nature is at the heart of a new crystalline material with intriguing properties.
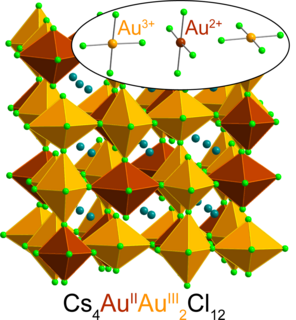
For the first time, Stanford researchers have found a way to create and stabilize an extremely rare form of gold that has lost two negatively charged electrons, denoted Au2+. The material stabilizing this elusive version of the valued element is a halide perovskite—a class of crystalline materials that holds great promise for various applications including more-efficient solar cells, light sources, and electronics components.
Surprisingly, the Au2+ perovskite is also quick and simple to make using off-the-shelf ingredients at room temperature.
"It was a real surprise that we were able to synthesize a stable material containing Au2+—I didn't even believe it at first," said Hemamala Karunadasa, associate professor of chemistry at the Stanford School of Humanities and Sciences and senior author of the study published Aug. 28 in Nature Chemistry. "Creating this first-of-its-kind Au2+ perovskite is exciting. The gold atoms in the perovskite bear strong similarities to the copper atoms in high-temperature superconductors, and heavy atoms with unpaired electrons, like Au2+, show cool magnetic effects not seen in lighter atoms."
"Halide perovskites possess really attractive properties for many everyday applications, so we've been looking to expand this family of materials," said Kurt Lindquist, the lead author of the study who conducted the research as a Stanford doctoral student and is now a postdoctoral scholar in inorganic chemistry at Princeton University. "An unprecedented Au2+ perovskite could open some intriguing new avenues."
Heavy electrons in gold
As an elemental metal, gold has long been valued for its relative scarcity as well as its unmatched malleability and chemical inertness—meaning it can be easily shaped into jewelry and coins that do not react with chemicals in the environment and tarnish over time. An additional key reason for its value is gold's namesake color; arguably no other metal in its pure state has such a distinctively rich hue.
The fundamental physics behind gold's acclaimed appearance also explains why Au2+ is so rare, Karunadasa explained.
The root reason is relativistic effects, originally postulated in Albert Einstein's famed theory of relativity. "Einstein taught us that when objects move very fast and their velocity approaches a significant fraction of the speed of light, the objects get heavier,” Karunadasa said.
This phenomenon applies to particles, too, and has profound consequences for “massive” heavy elements, such as gold, whose atomic nuclei boast a large number of protons. These particles collectively exert immense positive charge, forcing negatively charged electrons to whirl around the nucleus at breakneck speeds. As a consequence, the electrons grow heavy and tightly surround the nucleus, blunting its charge and allowing outer electrons to drift farther than in typical metals. This rearrangement of electrons and their energy levels leads to gold absorbing blue light and therefore appearing yellow to our eye.
Because of the arrangement of gold's electrons, thanks to relativity, the atom naturally occurs as Au1+ and Au3+, losing one or three electrons, respectively, and spurning Au2+. (The “2+” indicates a net positive charge from the loss of two negatively charged electrons, and the "Au" chemical symbol for gold hails from “aurum,” the Latin word for gold.)
A squeeze of vitamin C
With just the right molecular configuration, Au2+ can endure, the Stanford researchers found. Lindquist said he "stumbled upon" the new Au2+-harboring perovskite while working on a broader project centered on magnetic semiconductors for use in electronic devices.
Lindquist mixed a salt called cesium chloride and Au3+-chloride together in water and added hydrochloric acid to the solution "with a little vitamin C thrown in," he said. In the ensuing reaction, vitamin C (an acid) donates a (negatively charged) electron to the common Au3+ forming Au2+. Intriguingly, Au2+ is stable in the solid perovskite but not in solution.
"In the lab, we can make this material using very simple ingredients in about five minutes at room temperature," said Lindquist. "We end up with a powder that's very dark green, nearly black, and is surprisingly heavy because of the gold it contains."
Recognizing that they may have hit new chemistry paydirt, so to speak, Lindquist performed numerous tests on the perovskite, including spectroscopy and X-ray diffraction, to investigate how it absorbs light and to characterize its crystal structure. Stanford research groups in physics and chemistry led by Young Lee, professor of applied physics and of photon science, and Edward Solomon, the Monroe E. Spaght Professor of Chemistry and professor of photon science, further contributed to studying the behavior of Au2+.
The experiments ultimately bore out the presence of Au2+ in a perovskite and, in the process, added a chapter to a century-old story of chemistry and physics involving Linus Pauling, who received the Nobel Prize in Chemistry in 1954 and the Nobel Peace Prize in 1962. Early in his career, he worked on gold perovskites containing the common forms Au1+ and Au3+. Coincidentally, Pauling also later studied the structure of vitamin C—one of the ingredients required to yield a stable perovskite containing the elusive Au2+.
"We love Linus Pauling’s connection to our work," Karunadasa said. "The synthesis of this perovskite makes for a good story."
Looking ahead, Karunadasa, Lindquist, and colleagues plan to study the new material further and tweak its chemistry. The hope is that an Au2+ perovskite can be used in applications that require magnetism and conductivity as electrons hop from Au2+ to Au3+ in the perovskite.
"We're excited to explore what an Au2+ perovskite could do," Karunadasa said.
Karunadasa is also a senior fellow at the Precourt Institute for Energy and a principal investigator and faculty scientist at the Stanford Institute for Materials and Energy Sciences, SLAC National Accelerator Laboratory. Solomon is a professor of photon science at Stanford Synchrotron Radiation Lightsource, SLAC. Additional Stanford co-authors are Christina R. Deschene and Alexander J. Heyer, graduate students in the Department of Chemistry; and Jiajia Wen, a staff scientist at SLAC. Additional co-authors include Armin Eghdami and Alexander G. Smith, graduate students in the Department of Physics, University of California-Berkeley, and Jeffrey B. Neaton, professor of physics at the University of California-Berkeley; and Dominic H. Ryan, professor of physics at McGill University.
The research was funded in part by the U.S. National Science Foundation, the U.S. Department of Energy, the Fonds de recherche du Québec–Nature et technologies, and the Natural Sciences and Engineering Research Council Canada.
Media Contact: Holly Alyssa MacCormick, Stanford School of Humanities and Sciences: hollymac [at] stanford [dot] edu (hollymac[at]stanford[dot]edu)